















Do you want to contribute by writing guest posts on this blog?
Please contact us and send us a resume of previous articles that you have written.
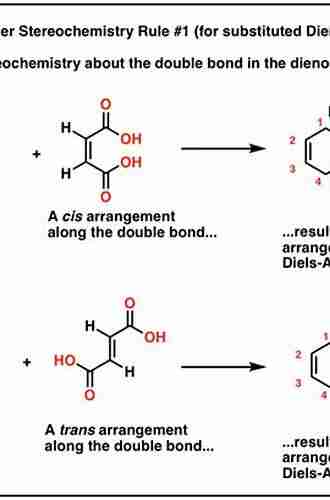
The Mind-Blowing Connection: Stereochemistry And Organic Reactions

Prepare to be amazed by the fascinating world of stereochemistry and organic reactions. Brace yourself for an in-depth exploration of how atoms interact and arrange themselves in space in organic compounds, and how these arrangements influence the chemical reactions that take place. Discover the secrets behind the mind-blowing complexity and precision of organic chemistry with this comprehensive guide.
Understanding Stereochemistry
Stereochemistry is the study of the three-dimensional arrangement of atoms in molecules and the effects of this arrangement on chemical reactivity. In organic chemistry, the arrangement of atoms in space is crucial to understanding how molecules interact and undergo various types of reactions.
Imagine holding your hands up with fingers spread apart. Even though both hands have the same number of fingers, they are mirror images of each other. Similarly, in chemistry, molecules can have mirror images called enantiomers. These enantiomers may look similar on paper, but their three-dimensional arrangements can result in drastically different biological and chemical properties.
4.1 out of 5
| Language | : | English |
| File size | : | 97377 KB |
| Text-to-Speech | : | Enabled |
| Screen Reader | : | Supported |
| Enhanced typesetting | : | Enabled |
| Print length | : | 640 pages |
| Hardcover | : | 440 pages |
| Item Weight | : | 1.6 pounds |
| Dimensions | : | 6.14 x 0.94 x 9.21 inches |
For example, consider the infamous thalidomide tragedy of the 1960s. Thalidomide was initially marketed as a sedative and antiemetic drug for pregnant women, but it caused severe birth defects. The drug existed as a racemic mixture, meaning it contained both enantiomers. While one enantiomer had the desired therapeutic effects, the other enantiomer caused devastating birth defects. This highlights the critical importance of stereochemistry in drug development and emphasizes the need to study the interactions between drugs and their biological targets.
Chirality and Optical Activity
The property that distinguishes enantiomers is chirality. A molecule is chiral if it lacks an internal plane of symmetry, meaning it cannot be superimposed on its mirror image. Chiral molecules often exhibit a phenomenon called optical activity, whereby they rotate the plane of polarized light. This property becomes extremely useful in determining the presence of chiral compounds and measuring their enantiomeric excess.
To visualize chirality, think of your hands again. No matter how you orient your hands in space, they cannot fully overlap. You can try it yourself, but you will never achieve perfect alignment. Chiral molecules behave in the same way. Their mirror images will never perfectly align.
The importance of chirality extends beyond pharmaceuticals. In the fragrance industry, for instance, some enantiomers of certain compounds contribute to pleasant smells, while their mirror images might produce unpleasant odors. The presence of chiral compounds even affects the flavors of food and beverages, as well as the effectiveness of crop protection agents in agriculture.
Organic Reactions and Stereochemistry
Now that we have a basic understanding of stereochemistry, let's explore how it impacts organic reactions. The three-dimensional arrangement of atoms in a molecule can significantly affect the reaction's outcome. Certain reactions are highly stereospecific, meaning they only occur with one stereoisomer but not the other.
For instance, enzymes, the biological catalysts, are highly selective for one enantiomer over the other. This phenomenon is crucial for catalytic reactions that occur in living organisms, ensuring the specificity and functionality of vital biochemical pathways. Understanding the stereochemistry of these reactions unlocks the potential for designing more efficient drugs that target specific enantiomers or for developing new catalysts inspired by enzymatic systems.
In addition to stereospecific reactions, stereochemistry plays a role in determining reaction rates and transition states. The spatial arrangement of functional groups in a molecule can facilitate or hinder the approach of other molecules, leading to varying reaction rates. Understanding these factors helps chemists optimize reaction conditions and improve the efficiency of synthesis.
Advanced Techniques and Spectroscopy
As technology advances, so does our ability to investigate stereochemistry and organic reactions. Modern spectroscopic methods, such as NMR, IR, and circular dichroism (CD),provide invaluable tools for analyzing molecular structures and elucidating reaction mechanisms.
Nuclear Magnetic Resonance (NMR) spectroscopy is particularly useful in distinguishing different enantiomers and determining their concentrations. It allows chemists to investigate the spatial arrangement of atoms within molecules, providing a deeper understanding of their three-dimensional structures.
Infrared (IR) spectroscopy complements NMR by providing information about functional groups present in a molecule. By analyzing the absorption of infrared radiation, chemists can infer the presence of specific bonds or groups that contribute to the stereochemistry of a compound.
Circular dichroism (CD) spectroscopy measures the differences in absorbance of left- and right-circularly polarized light. It is a powerful technique for analyzing chiral compounds as it can provide insight into their absolute configuration and quantify the enantiomeric excess present in a sample.
Stereochemistry is not just a concept confined to the realm of organic chemistry textbooks. It is a fundamental aspect of the molecular world, governing the behavior of molecules and shaping the outcome of chemical reactions. Understanding stereochemistry is crucial for various scientific fields, from drug discovery to fragrance design.
This article has only scratched the surface of stereochemistry and organic reactions. It is a vast and ever-evolving field, continuously expanding our knowledge and pushing the boundaries of what is possible. So next time you encounter a chiral compound, remember the intricate dance of atoms in space that gives rise to its unique properties and the amazing world of stereochemistry it represents.
4.1 out of 5
| Language | : | English |
| File size | : | 97377 KB |
| Text-to-Speech | : | Enabled |
| Screen Reader | : | Supported |
| Enhanced typesetting | : | Enabled |
| Print length | : | 640 pages |
| Hardcover | : | 440 pages |
| Item Weight | : | 1.6 pounds |
| Dimensions | : | 6.14 x 0.94 x 9.21 inches |
Stereochemistry and Organic Reactions: Conformation, Configuration, Stereoelectronic Effects and Asymmetric Synthesis provides coverage on the stereochemistry of reactions of all mechanistic types, ranging from ionic, pericyclic and transition metal-catalyzed to radical and photochemical. Chapters cover acyclic molecules, cyclic molecules, the stereochemistry of organic reactions, the perturbation molecular orbital theory for the origin of stereoelectronic effects, and an to the principles of stereoselectivity and hierarchical levels of asymmetric synthesis. Each chapter includes problems that reinforce main themes, making it valuable to students, teachers and researchers working in organic, biological and medicinal chemistry, as well as biologists, pharmacologists, polymer chemists and chemists.
- Presents a holistic and unified approach to stereochemical understanding and predictions, covering reactions of all mechanistic classes
- Includes two background chapters on perturbation theory and stereoselective principles, along with asymmetric designs
- Features novel rules and mnemonics to delineate product stereochemistry
- Includes up-to-date coverage with over 1300 selective references

 Grayson Bell
Grayson BellWellington's Incredible Military and Political Journey: A...
When it comes to military and political...

 Kenzaburō Ōe
Kenzaburō Ōe10 Mind-Blowing Events That Take Place In Space
Welcome to the fascinating world of...

 Joseph Conrad
Joseph ConradThe Astonishing Beauty of Lanes Alexandra Kui: Exploring...
When it comes to capturing the essence of...

 Arthur C. Clarke
Arthur C. ClarkeUnlock the Secrets of Riding with a Twist Of The Wrist
Are you a motorcycle...

 Clay Powell
Clay PowellThe Ultimate Guide to An Epic Adventure: Our Enchanting...
Are you ready for a truly mesmerizing and...

 Ashton Reed
Ashton ReedThe Last Great Revolution: A Transformation That Shaped...
Throughout history, numerous revolutions have...

 Julio Cortázar
Julio CortázarThe Cinder Eyed Cats: Uncovering the Mysteries of Eric...
Have you ever come across a book that takes...

 Theodore Mitchell
Theodore MitchellDiscover the Ultimate Spiritual Solution to Human...
In today's fast-paced, modern...

 Tony Carter
Tony CarterContract Law Made Easy Vol.: A Comprehensive Guide for...
Are you confused about the intricacies of...

 Jackson Blair
Jackson BlairThe Wright Pages Butterbump Lane Kids Adventures: An...
In the magical world of...

 Reginald Cox
Reginald CoxAmerica Nightmare Unfolding In Afghanistan
For more than two decades,...

 Sidney Cox
Sidney CoxCivil Rights Leader Black Americans Of Achievement
When it comes to the civil...
Light bulbAdvertise smarter! Our strategic ad space ensures maximum exposure. Reserve your spot today!

 Samuel BeckettThe Grand Exposition of Essays in Honour of Carl Chiarella - Unraveling the...
Samuel BeckettThe Grand Exposition of Essays in Honour of Carl Chiarella - Unraveling the...
 Stanley BellThe Ultimate Pet Guide: Everything You Need to Know about Species-Appropriate...
Stanley BellThe Ultimate Pet Guide: Everything You Need to Know about Species-Appropriate...
 Rodney ParkerEnchanted: The Woodcutter Sisters - A Magical Journey Through the Enchanted...
Rodney ParkerEnchanted: The Woodcutter Sisters - A Magical Journey Through the Enchanted... Roger TurnerFollow ·4.9k
Roger TurnerFollow ·4.9k Ross NelsonFollow ·15.5k
Ross NelsonFollow ·15.5k Oscar BellFollow ·11.7k
Oscar BellFollow ·11.7k Beau CarterFollow ·14.2k
Beau CarterFollow ·14.2k Asher BellFollow ·17.7k
Asher BellFollow ·17.7k Jace MitchellFollow ·11.9k
Jace MitchellFollow ·11.9k Chandler WardFollow ·17k
Chandler WardFollow ·17k Jack PowellFollow ·11.7k
Jack PowellFollow ·11.7k